Menlo Systems product:
Original publication:
Making movies of brain synapses
American and Czech scientists developed a novel microscope for deep brain imaging at speeds matching neural signaling.
Two-photon fluorescence microscopy is an effective method to reduce the amount of scattered light during brain imaging, enabling access to deeper tissue regions. However, raster-scanning of larger volumes requires long acquisition times making the imaging too slow to monitor a rapid signal migrating across the cortical space. A group of American and Czech scientists recently developed a new two-photon imaging technique enabling the acquisition of more than 1.4 x 109 voxels (i.e. volume elements) per second. By scanning lines of excitation across a focal plane at multiple angles, and by using prior information about the sample’s structure, they computationally reconstruct neural activity at high resolution. Menlo Systems’ BlueCut Femtosecond Fiber Laser serves as the excitation source. The group demonstrates the effectiveness of the novel imaging method in vitro and also in vivo in the visual cortex of mice, at more than 250 µm depth and frame rates larger than 1 kHz. The ability to image patterns of neural activity synchronized over longer distances allows visualizing information flow in a mammalian brain.
In neuroscience, specialized deep tissue imaging is used to record activity in the intact brain. Neuroscientists use microscopes to record spatiotemporal activity patterns of neuronal cells or synapses, and their response to external stimuli in vivo, to better understand cognitive states such as attention, task performance, as well as sleep or anesthesia. Their detailed knowledge nurtures the hope to be able to intervene or control them, e.g. for medical applications. Two-photon fluorescence microscopy gives visual access to up to few hundreds of micrometers depth in the brain. With this technique, the nonlinear excitation of fluorescent sensors confines measurements to the focus of the laser beam, rejecting background signals from above and below the focus. Simultaneously, the infrared wavelengths used for nonlinear excitation penetrate deeper into tissue than visible light. In order to image neuronal signals as they migrate over distances, or synchronous neural activity, the image frames need to encompass larger contiguous areas up to the millimeter scale. Point-wise voxel (i.e. volume element) acquisition as in raster scanning have to be performed sequentially, limiting the frame rate to tens of Hertz. The technique is therefore too slow to monitor fast migrating signals, such as action potentials, or temporal signal coherence.
A group of American and Czech scientists presents a novel two-photon microscope which is capable of acquiring images of high spatial resolution, over large volumes, and at kilohertz frame rates. The technique allows imaging of events occurring in synapses of individual neurons on the millisecond time scale and over hundreds of micrometers distance. Moreover, due to its non-destructive character, it can be applied in the intact brain. Being a computational image reconstruction technique, the new method combines benefits from other techniques such as random-access imaging and tomography. Using prior information such as the structural sample image or the fluorescent indicator model dynamics, the group is able to maintain the high resolution of the image.
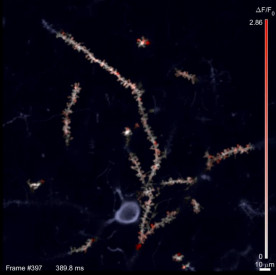
Figure: Snapshot frame of an activity recording from the SLAP microscope. Active synapses show up as red spots, inactive regions are white. The microscope records over 1000 frames per second.
The group’s device for Scanned Line Angular Projection Microscopy (SLAP) uses Menlo Systems’ BlueCut Femtosecond Fiber Laser and performs line scans in the focal plane of the laser beam across the field of view (FOV) at four different angles. Due to the line geometry of the scanned focus, the frame time is proportional to the diameter d of the FOV in pixels, as opposed to the d2 dependence in raster scanning, leading to greatly increased frame rates. A spatial light modulator defines illumination patterns in order to select relevant regions within the FOV and to further reduce the overall illumination power on sensitive samples. The possibility of patterned illumination is the underlying principle in a random-access microscope.
After the group performs an assessment of the microscope’s ability to distinguish and track single particles, and to provide high-resolution and high-sensitivity 3D images of neural activity in motion, they validate their method on several examples representing typical applications in neuroscience. Firstly, they image cultured neurons with different response characteristics to light excitation. In the resulting images the fluorescence is assigned in space with high accuracy, even in densely intertwined cultures. Furthermore, the timing precision of events proves to be highly accurate. Secondly, the group images synaptic activity in mouse visual cortex in vivo by recording visually-evoked and spontaneous patterns of glutamate release on neuronal dendrites. SLAP is able to resolve patterns of neuron activity with an accuracy even higher than by raster scanning. For instance, it depicts activity patterns triggered by stimuli travelling across single neurons’ dendrites as they travel across the mouse’s retina. The high frame rate allows observing activity transients on a fast time scale and revealed how glutamate release is synchronized at different frequencies during anesthesia.
In summary, the new method for diffraction-limited in vivo imaging is ideal to study rapid signals such as neurotransmitter release over hundreds of micrometers FOVs. The patterns of glutamate activity the scientists recorded are helping them understand how neurons communicate to each other and perform computations.
Author: Patrizia Krok
Original publication:
A. Kazemipour et al.: Kilohertz frame-rate two-photon tomography; Nature Methods Vol. 16, p. 778 (2019)
